
Next Generation Oncology Screening
Liquid biopsy for detection of somatic mutations in circulating cell-free tumor DNA (ctDNA) from a blood sample
WHY LIQUID BIOPSY?
“Liquid biopsy” is a safe, non-invasive, highly sensitive and cost effective method of analyzing circulating tumor DNA (ctDNA) from the plasma of patients diagnosed with cancer or from individuals who may have cancer.
By analyzing ctDNA isolated from a patient’s blood, we can identify clinically relevant genomic alterations in ctDNA and match these alterations to targeted therapies and clinical trials.
| TISSUE BIOPSY | LIQUID BIOPSY |
|---|---|
| Invasive and expensive | Non-invasive with a good benefit/cost ratio. |
| Strongly dependent on tumor position | Less dependent on original tumor site since tumor from both primary and metastatic sites release DNA into the bloodstream |
| Limited representativeness of genomic heterogeneity | Better represents tumor heterogeneity |
| Difficult or impossible to perform tissue biopsy in some organs | Blood sampling is simple and always accessible |
| Not applicable if primary tumor has been removed or if the tumor cannot be easily visualized via imaging studies | Can be used after surgery for evaluation in the absence of detectable primary tumor or metastases |
| Insufficient amount of tissue may be obtained for immunohistochemical and genomic analysis | A few copies of mutant ctDNA are sufficient for analysis |
| Serial biopsies are difficult to tolerate | Patient can tolerate serial blood draws for evaluation; may lead to greater compliance |
| It often needs preparation, long waiting times and specialized surgery area | Fast turnaround times and sampling is always performable without any surgery |
| Useful tool for dynamic monitoring of the therapeutic response and resistance development |
MONITOR DRUG RESISTANCE
Identification of somatic mutations on circulating tumor DNA (ctDNA) for cancer monitoring or for early detection of cancer. OnkoGENETIKSTM Liquid Biopsy test is intended for patients who have been diagnosed with cancer or individuals with high risk for a suspected cancer.
MONITOR & SCAN

PATIENTS DIAGNOSED WITH CANCER
• Provide tumor profiling for precision medicine
• Monitor treatment efficacy and resistance
• Monitor disease progression and tumor evolution
• Monitor disease recurrence
• Help physician consider other treatment options when the patient is resistant to current therapies
PATIENTS WITH A SUSPECTED CANCER
• Analysis of potential somatic mutations in genes known to be altered in cancer
• In the case of familial predisposition to cancer, relevant cancer genes are investigated for the presence of somatic mutations in order to detect early disease
Overview of the general workflow for OnkoGenetiks™ Liquid Biopsy Analysis
The overall procedure starts with DNA isolation from blood samples obtained in PAXgene Blood ccfDNA Tubes. After library construction (using OnkoGenetiksTM designed gene panel) and target enrichment steps, NGS is performed by using Illumina NextSeq 550Dx sequencing platform. Analysis of raw sequencing data is carried out and detected variants can be interpreted with the Ingenuity®Variant Analysis (IVA) or QIAGEN Clinical Insight (QCI™) tools.
MONITOR TREATMENT EFFECTIVENESS
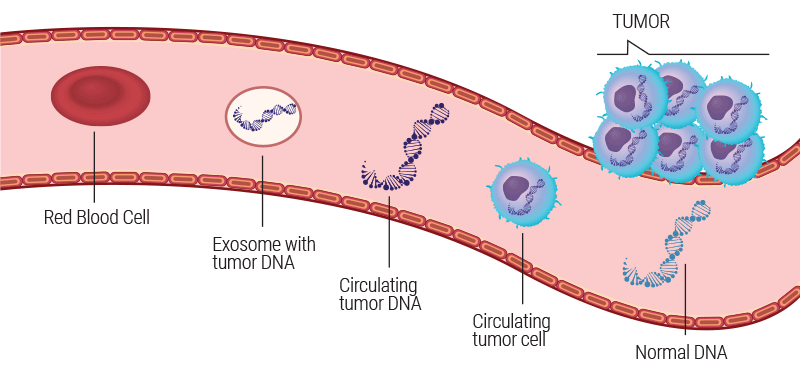
As healthy and diseased tissue interact with the bloodstream, they release both cellular and genetic material into the circulation. These materials are referred to as “cell-free” DNA (cfDNA), and circulating tumor cells (CTCs). Tumor DNA from these circulating tumor cells is termed as circulating tumor DNA (ctDNA).
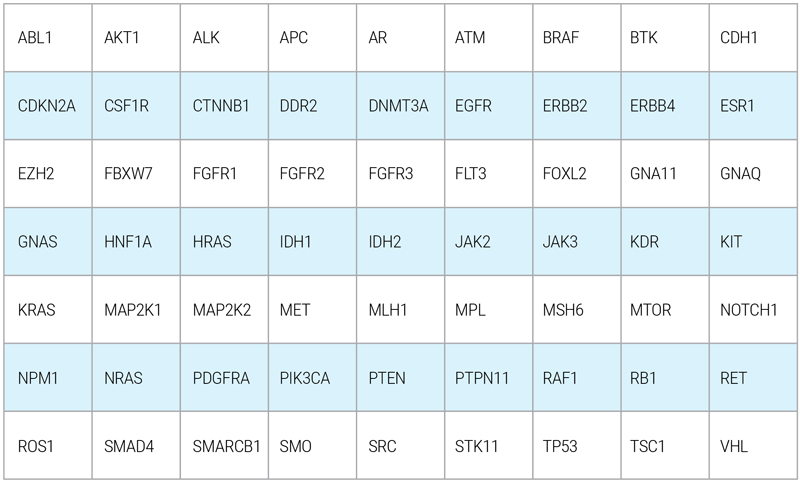
OnkoGenetiks™Liquid Biopsy gene panel covers a broad spectrum of high priority genes which are known to be most involved in cancer progression. The genes are stated as the following:
(Hotspot regions are being analyzed for certain genes)
Genetiks is accredited from CAP (College of American Pathologist) for Liquid Biopsy.

How to perform OnkoGenetiks™ LIQUID BIOPSY Test?



